BOOK 1: The Thought Process
19th March 2020: A plea, a football bladder and the wheel is mooted.
10:06: Akshay (The prime motivator): “This infection is probably going to be massive. I was wondering if there is some way we can contribute and help the medical community. A couple of days ago I saw a message from IISc colleague about making simple ventilators. We can probably reach out to medical community about what they might need and see how we can help.”
Team CeNSE had already been seized by the COVID problem since the 1st of March. After all they had the responsibility of running one of the largest clean rooms in the country and one of the most advanced in the world in an academic setting. A clean room is an air-conditioned space in which very expensive equipment is used to make electronic chips. If the clean room got contaminated and required fumigation crores of equipment would be at potential risk. They had discussed and debated on whether the facility should be put on lockdown. What if the pandemic did not pan out as it was predicted to? Would we be blamed for over-reacting? The research of 100s of students would be affected. What if it did? Would we not be blamed for being irresponsible?
They had been monitoring the situation unfolding in China and then in Italy closely. On the 8th of March, to smiles and the amusement of many, CeNSE had put out prominent posters and had started a sanitization protocol. On the 14th of March the Institute shutdown. On the 21st of March, the nation went into lockdown.
…And so it unfolded on the 19th of March 2020. A group of Professors in lockdown and wanting to do something. Not keep quiet.
They had been monitoring the situation unfolding in China and then in Italy closely. On the 8th of March, to smiles and the amusement of many, CeNSE had put out prominent posters and had started a sanitization protocol. On the 14th of March the Institute shutdown. On the 21st of March, the nation went into lockdown.
…And so it unfolded on the 19th of March 2020. A group of Professors in lockdown and wanting to do something. Not keep quiet.
11:13: Ambarish: “Absolutely. Let me find out from some of my doctor acquaintances if there is anything we can do.”
11:14: Vasu: “The issue is that both testing for COVID (that Sai works on) and such tools require certification. Navakant had commented on an email exchange regarding Sai yesterday. Something similar would have to be worked out here.”
11:26: Akshay: “While testing is important, I am guessing in these cases, they are going to relax those rules. e.g. is it better to not have a ventilator at all or do we build one that has some probability of working. We either let a person die or give them half a chance…”
11:27: Vasu: “I don’t disagree. Come up with a plan of action and let me know how I can help.”
12:05: Prosenjit: “TCS engineering services at Mysore was developing a ventilator prototype a few years ago. Maybe they can provide some help. What I remember from that interaction is that there are a few critical requirements other than the right gas composition. Pressure control, Sensing the breathing rate to synchronise the ventilator, Humidity and temperature. I am sure there will be other requirements. If there is a medical expert who can guide the requirements, then we can definitely think about building one.”
12:06: Ambarish: “I heard back from Dr.Venkatesh (RGUHS) who suggested meeting certain folks, who can frame the problem properly. But there must be something other than ventilators”
12:15: Akshay: Italy ran out of some parts that were manufactured using 3D printer.
I am sure people like Prof. Krishnaswamy would be very helpful for testing…
12:24: Akshay: Made a onenote note book. Maybe we can discuss ideas there.
14:01: Vasu: Subject: Ultimate Medical Hackathon: How Fast Can We Design And Deploy An Open Source Ventilator? | Hackaday
11:14: Vasu: “The issue is that both testing for COVID (that Sai works on) and such tools require certification. Navakant had commented on an email exchange regarding Sai yesterday. Something similar would have to be worked out here.”
11:26: Akshay: “While testing is important, I am guessing in these cases, they are going to relax those rules. e.g. is it better to not have a ventilator at all or do we build one that has some probability of working. We either let a person die or give them half a chance…”
11:27: Vasu: “I don’t disagree. Come up with a plan of action and let me know how I can help.”
12:05: Prosenjit: “TCS engineering services at Mysore was developing a ventilator prototype a few years ago. Maybe they can provide some help. What I remember from that interaction is that there are a few critical requirements other than the right gas composition. Pressure control, Sensing the breathing rate to synchronise the ventilator, Humidity and temperature. I am sure there will be other requirements. If there is a medical expert who can guide the requirements, then we can definitely think about building one.”
12:06: Ambarish: “I heard back from Dr.Venkatesh (RGUHS) who suggested meeting certain folks, who can frame the problem properly. But there must be something other than ventilators”
12:15: Akshay: Italy ran out of some parts that were manufactured using 3D printer.
I am sure people like Prof. Krishnaswamy would be very helpful for testing…
12:24: Akshay: Made a onenote note book. Maybe we can discuss ideas there.
14:01: Vasu: Subject: Ultimate Medical Hackathon: How Fast Can We Design And Deploy An Open Source Ventilator? | Hackaday
14:36: Prosenjit: AIIMS has developed a cheap ventilator… It might be a good starting point….
Other links for DIY ventilators :
14:55: Akshay: It is just a compressor with valve. Why would that be so expensive?
The MIT paper shows a balloon with mechanical arm to push air into lungs.
That seems like an easy thing too. Infact you can buy these kinds of things for a few thousand rupees.
The MIT paper shows a balloon with mechanical arm to push air into lungs.
That seems like an easy thing too. Infact you can buy these kinds of things for a few thousand rupees.
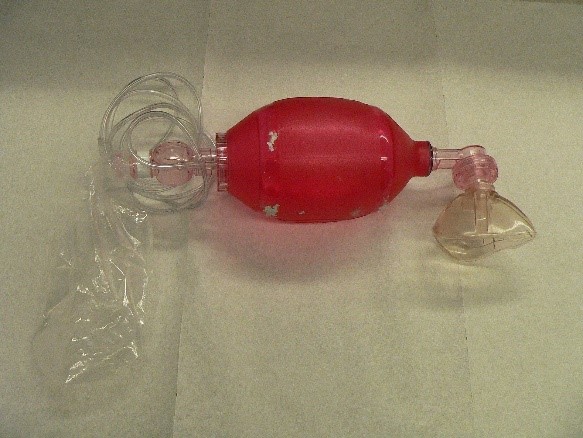
15:06: Ambarish: Football bladders are cheap and durable
15:11: Akshay: Should we try it? Lets at least ask Venkatesh about it…
6 hours had gone by. Akshay had not heard from anybody. He was probably getting frustrated.
15:11: Akshay: Should we try it? Lets at least ask Venkatesh about it…
6 hours had gone by. Akshay had not heard from anybody. He was probably getting frustrated.
21:08: Akshay: What do we do next?
Stuck in Vasu’s Draft folder: Soch raha hoon. Kal ek Skype call karte hain. Till then find out where we can get bellows from and how to automate it. We will then have to find out who manufactures it. Bellows ke handle pe ek spring mechanism lagana hoga to get it to suck. Then
.. and do it started with bellows and bladders!!
21:49: Vasu:
• OK, here you go. Just to get us started in response to Akshay’s passionate plea. I had to do something.
• Do we need it? Akshay to justify.
• If we expect to need it, here is something to get us started. Please feel free to add. I have put down tentative names against specific bits that might be needed. There are by no means no boundaries. Please feel free to comment. I have uploaded slides on one drive. Comment in the space below with you name.
• We mav have to meet online once to decide on possible designs that I would like the automation team to suggest based on these elements and what are required.
• OK, here you go. Just to get us started in response to Akshay’s passionate plea. I had to do something.
• Do we need it? Akshay to justify.
• If we expect to need it, here is something to get us started. Please feel free to add. I have put down tentative names against specific bits that might be needed. There are by no means no boundaries. Please feel free to comment. I have uploaded slides on one drive. Comment in the space below with you name.
• We mav have to meet online once to decide on possible designs that I would like the automation team to suggest based on these elements and what are required.
• Shall we meet tomorrow evening at 7:00 pm?
The first discussion slide set on the various elements the team would have to grapple with
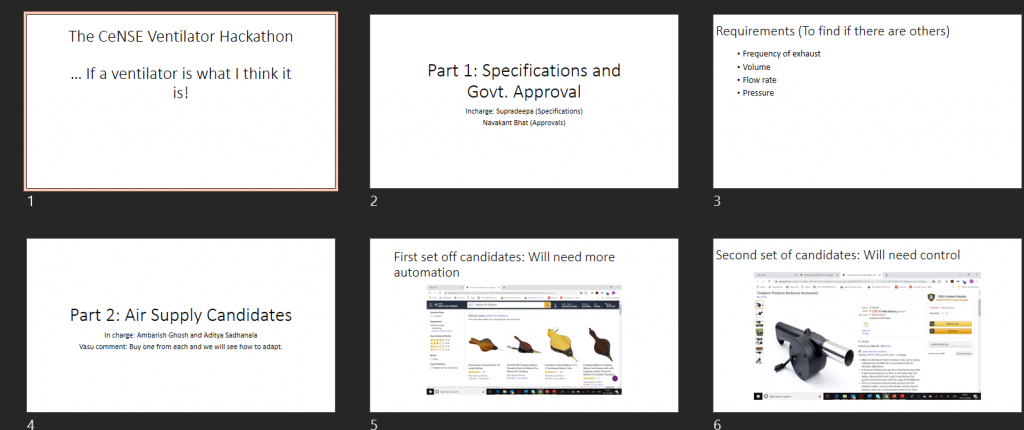
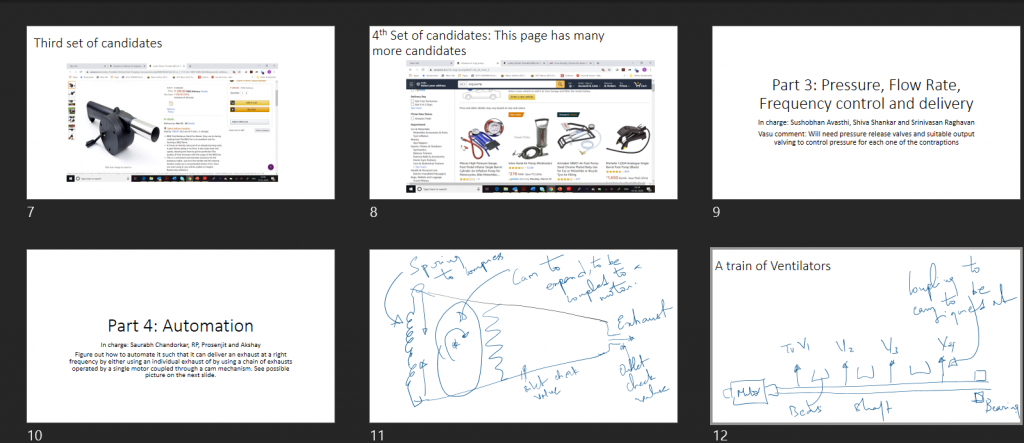



20th March: Should we do it, should we not do it?
11:21: Akshay: I talked to my brother about the ventilators. He said even if you build it, doctors won’t use it because it is not govt approved. As I said earlier, liability issues won’t matter much if they have to choose between giving something and giving nothing. Central govt can in emergencies approve the use of medical equipment without the required test but govt has not moved on it (as far as I know). Companies can probably ramp up production of approved ones but govt hasn’t said anything to them. There are obviously smaller companies with slightly cheaper price like the one Prosenjit mentioned but again that needs govt approval. Apparently we have only about 2000 ventilators in Karnataka. At this rate of infection, India would need 1 lakhs by the end of April. Has govt thought about it? Atleast I have no idea. We should push PSA office to at least get the manufacturers to ramp up the production and fast track approval of smaller ones. The other simpler thing that can be done is personal protective equipment. We would run short of them quickly. These are body suits, face shields and masks. I am not sure what the status is in India.
11:30: Saurabh: Since we are being serious about this, I have a few basic queries to make. I did do some research online. But, as we all know, if we are talking about a product, we have to hear it from the horse's mouth (in this case some doctors with the right knowledge). Do we have a good idea about the design parameters of a ventilator? Would it be possible to get an input from a doctor who specializes in these systems to actually know what the requirements are? I checked out a few specifications online. However, it is difficult to ascertain whether a given feature is a frill or not. Basically, what we have to start with is "What are the essential functionalities and specifications for a patient suffering from Covid19?" The fact is that India has several manufacturers of Ventilators. The super cheap one is priced at 20kRs. I am not sure if this one has the approval. If it is, then it will be hard to beat this cost no matter what we do here. On the other hand, if doctors don't like the 20k version, we need to find out what they don't like about it.
11:49: Navakant: Dear Vasu and others, (Authors note: Navakant is the chair of the department has commercialized a Bio Med device. He is genuinely worried, and correctly so, that his friends will be unnecessarily wasting their time”)
What Akshay mentioned is on the dot and is very important to recognize that it is the norm in healthcare systems anywhere in the world. The approval process is easier said than done, having experienced its convoluted pathways firsthand, over the last few years. Even when there is a major crisis, the doctors would want to go with the approved gadgets, no matter how expensive they are, rather than experimenting with new unapproved gadgets. Take the simple example of diagnostics/testing as opposed to medical intervention/treatment (which is more serious and such devices get classified as Class C or Class D). We wanted to fast track validation of another such invention, but it is already facing tough hurdles even to get the doctors experiment with it. The health community says that they are fighting a war now, and they simply do not have time and energy for any other distractions. They do not want to experiment with anything new even for testing. They would rather buy new expensive kits which are approved (the multinational Roche is the most recent example, which is not scaling up its approved test kits). Only when the current epidemic comes under control (perhaps after couple of months), with this other inventor’s work will be taken up as a research project to validate and possibly to prepare well for the next wave of Corona, which experts say is likely to hit in the winter this year.
In my opinion, all these efforts need to wait for few months, until we are on the other side of the current epidemic. The best way to contribute at this time is to leverage where we can contribute right now (such as the voluntary decision of CeNSE to make sanitizer and provide it to the entire Institute community), and strictly cooperate with the government on "social isolation" and related measures, sensitize others to follow such guidelines voluntarily rather than waiting for government to come down with sever restrictions (like what California did today). We can take up new research interventions once the current wave flattens out:
To quote Science editorial shared by Arunan:
"As for the scientific community who are not working on the virus—we know well that other major problems still exist, such as climate change, inequality, and other diseases. It is understandably very difficult to pause research in other arenas for an indefinite amount of time. This crisis is calling for extraordinary measures, and your supportive responses deserve recognition. Working from home will make it safer for those who must be in buildings and laboratories to do work related to the virus—fewer people in the hallways, lunchrooms, and other public areas will slow the spread of the virus so that work on COVID-19 can continue. If there is a way for you to assist without slowing these labs, volunteer to do so. If you have colleagues who are working on the virus, an offer of your time to keep an eye on their children or call upon their elderly relatives who are lonely can make a difference."
11:30: Saurabh: Since we are being serious about this, I have a few basic queries to make. I did do some research online. But, as we all know, if we are talking about a product, we have to hear it from the horse's mouth (in this case some doctors with the right knowledge). Do we have a good idea about the design parameters of a ventilator? Would it be possible to get an input from a doctor who specializes in these systems to actually know what the requirements are? I checked out a few specifications online. However, it is difficult to ascertain whether a given feature is a frill or not. Basically, what we have to start with is "What are the essential functionalities and specifications for a patient suffering from Covid19?" The fact is that India has several manufacturers of Ventilators. The super cheap one is priced at 20kRs. I am not sure if this one has the approval. If it is, then it will be hard to beat this cost no matter what we do here. On the other hand, if doctors don't like the 20k version, we need to find out what they don't like about it.
11:49: Navakant: Dear Vasu and others, (Authors note: Navakant is the chair of the department has commercialized a Bio Med device. He is genuinely worried, and correctly so, that his friends will be unnecessarily wasting their time”)
What Akshay mentioned is on the dot and is very important to recognize that it is the norm in healthcare systems anywhere in the world. The approval process is easier said than done, having experienced its convoluted pathways firsthand, over the last few years. Even when there is a major crisis, the doctors would want to go with the approved gadgets, no matter how expensive they are, rather than experimenting with new unapproved gadgets. Take the simple example of diagnostics/testing as opposed to medical intervention/treatment (which is more serious and such devices get classified as Class C or Class D). We wanted to fast track validation of another such invention, but it is already facing tough hurdles even to get the doctors experiment with it. The health community says that they are fighting a war now, and they simply do not have time and energy for any other distractions. They do not want to experiment with anything new even for testing. They would rather buy new expensive kits which are approved (the multinational Roche is the most recent example, which is not scaling up its approved test kits). Only when the current epidemic comes under control (perhaps after couple of months), with this other inventor’s work will be taken up as a research project to validate and possibly to prepare well for the next wave of Corona, which experts say is likely to hit in the winter this year.
In my opinion, all these efforts need to wait for few months, until we are on the other side of the current epidemic. The best way to contribute at this time is to leverage where we can contribute right now (such as the voluntary decision of CeNSE to make sanitizer and provide it to the entire Institute community), and strictly cooperate with the government on "social isolation" and related measures, sensitize others to follow such guidelines voluntarily rather than waiting for government to come down with sever restrictions (like what California did today). We can take up new research interventions once the current wave flattens out:
To quote Science editorial shared by Arunan:
"As for the scientific community who are not working on the virus—we know well that other major problems still exist, such as climate change, inequality, and other diseases. It is understandably very difficult to pause research in other arenas for an indefinite amount of time. This crisis is calling for extraordinary measures, and your supportive responses deserve recognition. Working from home will make it safer for those who must be in buildings and laboratories to do work related to the virus—fewer people in the hallways, lunchrooms, and other public areas will slow the spread of the virus so that work on COVID-19 can continue. If there is a way for you to assist without slowing these labs, volunteer to do so. If you have colleagues who are working on the virus, an offer of your time to keep an eye on their children or call upon their elderly relatives who are lonely can make a difference."
12:02: Shivashankar: About two months ago, my (older) sister had to be placed on a ventilator, in one of the "Super-Specialty" hospitals in Bengaluru. (My sister made through it.) I was there in the ICU when my brother-in-law had to sign the consent form. I asked the doctor present a lot of questions about the equipment, about the procedure. It was a Philips Ventilator, about 3 years old, and had the CE certification. I could not find out where it had been made. We need to find doctors who know much about ventilation, ventilators, and the procedures (processes) involved, who are interested in the mechanics of it. How and where do we find them?. I was about to raise the issue of approvals, but it has already been addressed very well in this thread.
20: 26 Vasu: But should we not start working on an inexpensive ventilator now? When it peters out, we will forget it and life will move on till the next round. If things are expected to blow up as they are expected to do and if a choice had to be made between no ventilator and some hack job, would we still not want to try it out?
20: 26 Vasu: But should we not start working on an inexpensive ventilator now? When it peters out, we will forget it and life will move on till the next round. If things are expected to blow up as they are expected to do and if a choice had to be made between no ventilator and some hack job, would we still not want to try it out?
Its late on a Friday night and their minds are still debating this problem..
21:29: Navakant: I hope you went through my other email where I had detailed about the story of SBMT funded ventilator project, which eventually made it into an indigenous product marketed by Skanray, with CE certification.
On your specific question of whether we should embark on a low cost ventilator development project, I am certainly not discouraging any of you, but let me state my views again:
1. Unlike in other fields, in healthcare the gestation period for an idea to be converted onto a product which can even be tested for its suitability is VERY LONG due to regulatory framework, rightly so because human health is involved
2. In other words there is a HUGE valley of death between Idealism and Pragmatism when it comes to healthcare.
3. If you start on a ventilator project now from scratch, it will be at least 2 years (or more) before it can even be presented for regulatory framework approval process. Believe me, you can not have it out in the field for the next few seasons of COVID, forget making any impact this season. After you initiate regulatory process, just one part of it is ISO 13485, which itself takes more than a year!
4. Finally, I personally am not convinced that a low cost ventilator is the pain point in the field. If government were to make contract with Skanray, for a large number of ventilators to be supplied tomorrow, I am sure the cost will be so low that you will not be able to beat it. Having said all this, if you guys still want to go ahead with ventilator hack, please do so. But you really need one champion who will stick with this idea for next 5 years (i.e. whose life depends on it, literally!)
22:47: Vasu: I did read. Under normal circumstances, I completely agree with what you are saying.
• It is just that these might be unprecedented circumstances. (I hope it does not come to it)
• The aim of this hack, is not to make a product that will beat Skanray who seem to be making a highly instrumented ICU ventilator. If Skanray can scale up, then they would be the best bet. However, even in Italy, apparently they had to make a choice between whom to keep alive and whom to not, just because a method of pushing air into the lungs was not available.
• I just happened to be reading up Wikipedia and the number of possible simple solutions are quite a few. One of them that was used in England in the 60’s comes very close to what I was envisioning it to be. A bellow that gets inflated by a compressor/oxygen cylinder and then, falls down under a weight. I am actually very surprised that in spite of many such simple available solutions, a shortage was experienced. One model is seen below. An even simpler solution is what we could come up with.
23:16; Vasu: Is it possible to find out what really the ventilator scarcity is about, if you know someone some ventilator manufacturer. Given how simple many ventilator designs are, unless everybody needed a highly instrumented ICU ventilator, it should not have resulted in a scarcity. If there were really so many cases requiring ICU type ventilators, then there is little hope.
On your specific question of whether we should embark on a low cost ventilator development project, I am certainly not discouraging any of you, but let me state my views again:
1. Unlike in other fields, in healthcare the gestation period for an idea to be converted onto a product which can even be tested for its suitability is VERY LONG due to regulatory framework, rightly so because human health is involved
2. In other words there is a HUGE valley of death between Idealism and Pragmatism when it comes to healthcare.
3. If you start on a ventilator project now from scratch, it will be at least 2 years (or more) before it can even be presented for regulatory framework approval process. Believe me, you can not have it out in the field for the next few seasons of COVID, forget making any impact this season. After you initiate regulatory process, just one part of it is ISO 13485, which itself takes more than a year!
4. Finally, I personally am not convinced that a low cost ventilator is the pain point in the field. If government were to make contract with Skanray, for a large number of ventilators to be supplied tomorrow, I am sure the cost will be so low that you will not be able to beat it. Having said all this, if you guys still want to go ahead with ventilator hack, please do so. But you really need one champion who will stick with this idea for next 5 years (i.e. whose life depends on it, literally!)
22:47: Vasu: I did read. Under normal circumstances, I completely agree with what you are saying.
• It is just that these might be unprecedented circumstances. (I hope it does not come to it)
• The aim of this hack, is not to make a product that will beat Skanray who seem to be making a highly instrumented ICU ventilator. If Skanray can scale up, then they would be the best bet. However, even in Italy, apparently they had to make a choice between whom to keep alive and whom to not, just because a method of pushing air into the lungs was not available.
• I just happened to be reading up Wikipedia and the number of possible simple solutions are quite a few. One of them that was used in England in the 60’s comes very close to what I was envisioning it to be. A bellow that gets inflated by a compressor/oxygen cylinder and then, falls down under a weight. I am actually very surprised that in spite of many such simple available solutions, a shortage was experienced. One model is seen below. An even simpler solution is what we could come up with.
23:16; Vasu: Is it possible to find out what really the ventilator scarcity is about, if you know someone some ventilator manufacturer. Given how simple many ventilator designs are, unless everybody needed a highly instrumented ICU ventilator, it should not have resulted in a scarcity. If there were really so many cases requiring ICU type ventilators, then there is little hope.
21st March: Ventilator Ditched!
10:23: Navakant: This is my one last attempt to convey some more facts.
I am doing this because, I know how important all of your time is, and I do not want your time and energy to be spent on things that are not useful...
In science, there is a danger of doing research without due diligent prior art search, which might result in reinventing the wheel with wasted resources. The same is true in research and development for any product. What I see here is jumping on something without due diligent search on what exists out there. The intentions are extremely noble, but the objective itself may be misplaced.
Let us forget Skanray. Now I want all of you to look at another Indian company which has productized the world's most economical ventilator.
They have worked with AIIMS for the last few years and developed one of its kind ventilator, with minimum bells and whistles, interfacing to Android phone (what Prof. SAS was talking about), everything that you want to imagine in a low cost ventilators. What is more important : their ventilator weighs less than 3 Kgs and it is portable! If you go to their website, now their tagline is : "FIGHT COVID-19 WITH WORLD'S MOST ECONOMICAL AGVA VENTILATORS".The crucial fact is that they already have ISO 13485! certificate and their ventilators are being used in several hospitals. They have even come forward, in these testing times, that they are willing to license their technology to any manufacturer to fight COVID. So what should we be doing now, under these testing times? Hack another ventilator or try and promote the ventilators like XX which cost less than Rs 50,000, fully certified for use for a COVID patient TODAY.
In fact if the government places an order for reasonably large number of ventilators the cost can even come down to less than Rs. 10,000. Cost is funny thing in business, it all depends on volumes...
I am doing this because, I know how important all of your time is, and I do not want your time and energy to be spent on things that are not useful...
In science, there is a danger of doing research without due diligent prior art search, which might result in reinventing the wheel with wasted resources. The same is true in research and development for any product. What I see here is jumping on something without due diligent search on what exists out there. The intentions are extremely noble, but the objective itself may be misplaced.
Let us forget Skanray. Now I want all of you to look at another Indian company which has productized the world's most economical ventilator.
They have worked with AIIMS for the last few years and developed one of its kind ventilator, with minimum bells and whistles, interfacing to Android phone (what Prof. SAS was talking about), everything that you want to imagine in a low cost ventilators. What is more important : their ventilator weighs less than 3 Kgs and it is portable! If you go to their website, now their tagline is : "FIGHT COVID-19 WITH WORLD'S MOST ECONOMICAL AGVA VENTILATORS".The crucial fact is that they already have ISO 13485! certificate and their ventilators are being used in several hospitals. They have even come forward, in these testing times, that they are willing to license their technology to any manufacturer to fight COVID. So what should we be doing now, under these testing times? Hack another ventilator or try and promote the ventilators like XX which cost less than Rs 50,000, fully certified for use for a COVID patient TODAY.
In fact if the government places an order for reasonably large number of ventilators the cost can even come down to less than Rs. 10,000. Cost is funny thing in business, it all depends on volumes...
At this point the email trail goes cold on the Ventilator. The team continues to discuss other problems they can work on such as masks, mask testing, mask disinfection, PPE and exhaust incinerators and so on.
By this point in time CeNSE has started making Sanitizers and supplying it essential services in the campus.
21st of March: 11:00 am: The team meets online to discuss what we should do.
It’s a Saturday! The families are unhappy that these guys are still working….
11:59: Akshay (In response to Navakant): I see your point...
How quickly can it be produced?
How many?
Do you know if govt has asked them to produce it in numbers that are needed?
Not saying we can do better or cheaper but hoping that at least a few lives can be saved if we run short in these.
If the data shows that we have or will have enough equipment at the peak demand then we should back off. Else we need to plan for a disaster.
This is where having clear information about preparedness would help which is severely lacking.
Maybe PSA office does have this information. If someone has access to him please do ask him.
11:52: Vasu: (Post the meeting) Ok. Given the inputs below, we decided to shelve the idea. I hope they will have enough and it will be worth it.
A doctor friend of my told me that when it comes to the point that somebody needs a ventilator in such epidemics they are given a “red card”!
How quickly can it be produced?
How many?
Do you know if govt has asked them to produce it in numbers that are needed?
Not saying we can do better or cheaper but hoping that at least a few lives can be saved if we run short in these.
If the data shows that we have or will have enough equipment at the peak demand then we should back off. Else we need to plan for a disaster.
This is where having clear information about preparedness would help which is severely lacking.
Maybe PSA office does have this information. If someone has access to him please do ask him.
11:52: Vasu: (Post the meeting) Ok. Given the inputs below, we decided to shelve the idea. I hope they will have enough and it will be worth it.
A doctor friend of my told me that when it comes to the point that somebody needs a ventilator in such epidemics they are given a “red card”!
Team decides to SHELVE the ventilator project!! Main considerations are that cost is not an issue and its production capacity that we cannot do anything about anyways. Team is glad to hear of initiatives from corporations like BEL, Maruti etc. to scale up production and feels that is the best way forward.
.. BUT cannot stop thinking…
15:38: Rudra Pratap: I had a chat with RK who, as you know, chairs the Bangalore cluster of the PSA office’s cluster initiative. RK was asking me about any initiative we have on ventilators. I told him of all the online discussion that we have had so far. I told him about Skanray and AgVa Healthcare. He made a good suggestion: can we find out from Skanray if they need any help in redesigning any part that may be a showstopper in terms of scaling up the production. Maybe we could help there if required. For example, maybe there is some part that can be done rapidly with many 3D printers, continuously printing it out. I told him that you might know the Skanray folks. Could you please talk to them and find out:
1. What is their production capacity?
2. Have they been contacted by any govt agency for scaling up their production?
3. What is their current unit price?
4. Do they need any help for scaling up production, especially if they think there is something that IISc can help them with?
20:00: (Mail stuck in Vasu’s folder. He has made some enquiries and finds out that there are no ventilators available for less than 2 lakhs. Dr. Justin Gopaldas tells him that they actually cost more than 5 lakhs typically and the best ones up to 25 lakhs. Saurabh confirms that this is indeed true.) BTW I just called some distributors. The minimum price is 2 lakhs. In case you find the low price source can you let me know?
1. What is their production capacity?
2. Have they been contacted by any govt agency for scaling up their production?
3. What is their current unit price?
4. Do they need any help for scaling up production, especially if they think there is something that IISc can help them with?
20:00: (Mail stuck in Vasu’s folder. He has made some enquiries and finds out that there are no ventilators available for less than 2 lakhs. Dr. Justin Gopaldas tells him that they actually cost more than 5 lakhs typically and the best ones up to 25 lakhs. Saurabh confirms that this is indeed true.) BTW I just called some distributors. The minimum price is 2 lakhs. In case you find the low price source can you let me know?
23rd March: The PHOENIX RIses….
8:23: Vasu to (Sushobhan, Saurabh and Akshay): Want to chat today. I have one meeting from 10:30 to 12:30. Else I am free.
9:07: (Its that) Akshay (Again): This MD is trying out these splitter valves. That way they can use the same ventilator for multiple people. A simple improvement would be to have valves that can control the flow so that different patients with different required flow rates can be catered to. I am guessing this should be fairly easy to do with manual valves..
9:21 Prosenjit: I guess a simple water tap kind of threaded valve can be designed and 3D printed quickly…
If they want a few splitters, we need to figure out how to get it done in our labs… I mean we need to get in contact with the student to see if he can help us in printing… need to contact the faculty for access to the 3D printers…
9:07: (Its that) Akshay (Again): This MD is trying out these splitter valves. That way they can use the same ventilator for multiple people. A simple improvement would be to have valves that can control the flow so that different patients with different required flow rates can be catered to. I am guessing this should be fairly easy to do with manual valves..
9:21 Prosenjit: I guess a simple water tap kind of threaded valve can be designed and 3D printed quickly…
If they want a few splitters, we need to figure out how to get it done in our labs… I mean we need to get in contact with the student to see if he can help us in printing… need to contact the faculty for access to the 3D printers…
3D printing was a saviour. Our ventilator could never have happened without a 3D Printer.
9:54 am: Vasu: I did not want to trouble everybody but Sushobhan, Akshay and I will be chatting today at 1:30 on ventilators and ventilation. Anybody who wants to join is welcome.
Author’s note: At this point in time team has decided they do not want to make a ventilator but simpler ventilation solutions (July 18:2020: It so turns out now that this is a solution that is required now! And not a ventilator). A “ventilation solution” as opposed to a ventilator is under discussion. The idea is to set up an industrial scale ventilation system in along the line of the CeNSE clean room gas supply system, but to supply air and oxygen mixtures. Subject of Vasu’s email reads: Ventilation and not Ventilator 23/3: 13:23
13:30: Team meets again!! Discusses simple ventilation ideas. The team debates ventilation with just cams again. Akshay suggests two cams out of phase are discussed to supply pressurise air and then allow the patient to exhale.
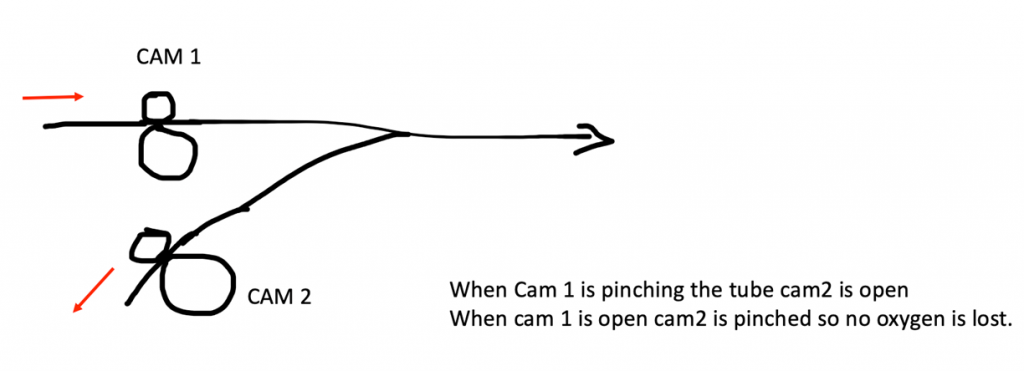
15:47: Akshay: (…and they simply just cannot stop thinking. “How do you solve a problem called an Engineeeeer?”) You were worried about that pinching with 3D printed parts.
Here are two ways we can mitigate that problem.
1. You don’t really need to pinch it very hard and completely cutoff the flow. There can be a small forward leak into the patient but that is required anyway.
2. Problem would be when patient exhales. You don’t want that to go back into the inlet. TO avoid this use one way valves in the lines behind the CAM. I know it is extra cost but ventilators use them a lot, so might be easy to get hold of them.
Here are two ways we can mitigate that problem.
1. You don’t really need to pinch it very hard and completely cutoff the flow. There can be a small forward leak into the patient but that is required anyway.
2. Problem would be when patient exhales. You don’t want that to go back into the inlet. TO avoid this use one way valves in the lines behind the CAM. I know it is extra cost but ventilators use them a lot, so might be easy to get hold of them.
20:22: Vasu:
Introductions:
IISc: Everybody on the first row. Sushobhan, Saurabh, Prosenjit, Akshay, RP and me (Vasu)
Manjunath: CEO of KAS Technologies
Justin: Doctor in Manipal Hospitals works in ICU.
(The first team is formed,…more will join later)
Hi all:Introductions:
IISc: Everybody on the first row. Sushobhan, Saurabh, Prosenjit, Akshay, RP and me (Vasu)
Manjunath: CEO of KAS Technologies
Justin: Doctor in Manipal Hospitals works in ICU.
The Doctor and Industrialist join the team.
Update: (Juxtaposing what Justin told me with what I know to present the status)
- Justin feels the contraption discussed is a good shot and similar to something he used on patients who were at 0% response.
- If I understood him correctly, a general patient will have between 0-100% response and the ideal ventilator should only make up for any shortfall. For instance if a patient is at 60% of normal capacity then the ventilator should do the remaining 40%. This is how an instrumented ventilator works. There is feedback from the body based on which the ventilator can adjust using PID loops.
- He said that if we come up with our contraption, which by the way has the variables he is looking for (pressure, high and low, volume and flow rate, frequency), he is willing to test it. His house is in Sanjaynagar.
- The pressure bit at the bottom will be challenging.
- Action plan: Let us sync up tomorrow given this data and confirmation by other members on this team and see how quickly we can put a plan together.
- He said that if we come up with our contraption, which by the way has the variables he is looking for (pressure, high and low, volume and flow rate, frequency), he is willing to test it. His house is in Sanjaynagar.
- The pressure bit at the bottom will be challenging.
- Action plan: Let us sync up tomorrow given this data and confirmation by other members on this team and see how quickly we can put a plan together.
The problem is better defined.
Data:
A. Number of breaths per minute:
1. birth to 6 weeks: 30–40 breaths per minute
2. 6 months: 25–40 breaths per minute
3. 3 years: 20–30 breaths per minute
4. 6 years: 18–25 breaths per minute
5. 10 years: 17–23 breaths per minute
6. Adults: 12-18 breaths per minute[9]
7. Elderly ≥ 65 years old: 12-28 breaths per minute.[12]
8. Elderly ≥ 80 years old: 10-30 breaths per minute.[12]
A. Number of breaths per minute:
1. birth to 6 weeks: 30–40 breaths per minute
2. 6 months: 25–40 breaths per minute
3. 3 years: 20–30 breaths per minute
4. 6 years: 18–25 breaths per minute
5. 10 years: 17–23 breaths per minute
6. Adults: 12-18 breaths per minute[9]
7. Elderly ≥ 65 years old: 12-28 breaths per minute.[12]
8. Elderly ≥ 80 years old: 10-30 breaths per minute.[12]
B. Volume: Typically about 12 breaths per minute at 500 ml per breath. Inhale to exhale time ratio is 1:1.5-2. Need more detail here and a better understanding of the graph below on minute (as in time) volume. Assuming a 5 second cycle and therefore a 1.66 second inhalation we are looking at a flow meter that will allow 0.5 liters in 1.66 seconds or 18 slpm flow rate range of rota meter.
Data below: Look up Wikipedia for details on “minute volume.”
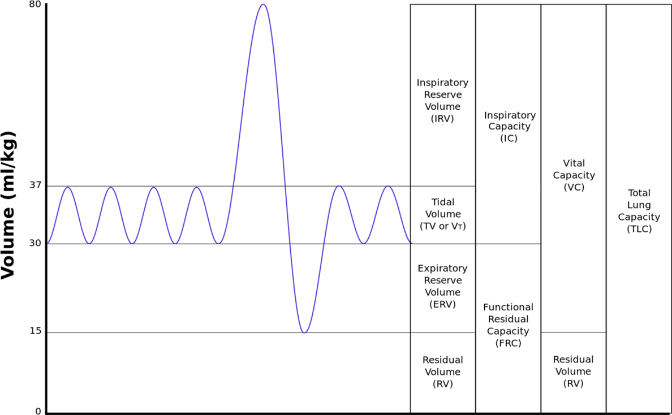
C. Pressure range: 12-30 cm of water column or 9-22 Torr gage pressure. This is a very small increment over atmosphere and needs careful control. Need to double check on this one.
23rd March: A CALL from Gaurab (Project PRANAA)
Vasu’s Note: I have often been asked as to why IISc had two ventilator projects running in parallel. Gaurab, a good friend, called me on the 23rd with queries on sourcing solenoids. This was a problem for all of us and we were in the midst of a lockdown. At that point the CeNSE team had actually decided to shelve the ventilator and go only for a simpler ventilation solution. Thus, apparently, we were working on two different things. As you will see later, once we made the first ventilation solution, the team could not stop. One thing led to another and before we knew it, we had another ventilator and the question. I am glad to note as of today that IISc has provided the country with two options. Congratulations to Team Pranna for a fabulous effort.
(Note:19th July 2020: As this story is being written the team is working on an electronics free ventilation solution!! Back to square one. This is apparently what is now needed for the COVID scenario. Not a ventilator! We hope our ventilators can still be certified and sold. We are in the process of doing that.)
